back to textbook
What is scale?
One might say that "scaling is the result of chemical precipitation becoming immobilized in a flowing system". In petroleum production, the produced water, gas and oil streams define the “flowing system”. These streams carry the chemical components that can precipitate.Scaling in production systems becomes a growing problem in reservoirs where the pressure balance is maintained by injection of waters that are not compatible with the formation waters, and the problem increases, as the reservoir grows more and more mature. In the North Sea most of the injection water has so far been composed of filtered and deoxygenated seawater. The seawater contains considerable amounts of sulphate (2-3000 ppm) while the formation waters often have a relatively high concentration of heavy alkaline earth metals calcium, strontium and barium. See table below for typical analysis of seawater ionic composition and a few examples of formation water compositions both from the North Sea and from other parts of the world.
Obvious from the examples given is that there may be a large variation in formation water salinity from one reservoir to another, and the relations between concentrations of the dissolved ions may change dramatically. Therefore, contact between injection and formation waters may entail precipitation and scale formation because the solubility products of the various actual chemical compounds may be superseded (see table below). The main components in scales are found to be BaSO4 (+ SrSO4) and CaCO3.
| Table 1: Concentration of the main ionic components in seawater and a few formation |
||||||||
|---|---|---|---|---|---|---|---|---|
| Ions |
Sea water mg/l1) |
Format. water I mg/l |
Format. water II mg/l |
Format. water III mg/l |
Format. water IV mg/l |
Format. water V mg/l |
Format. water VI mg/l |
Format. water VII mg/l |
| Cations: |
||||||||
| Na+ |
12.100 |
9.220 |
8.440 |
19.000 |
16.800 |
33.500 |
39.100 |
6.300 |
| K+ |
460 |
164 |
159 |
- |
24 |
554 |
1.750 |
330 |
| Mg2+ |
1.130 |
61 |
25 |
380 |
131 |
374 |
2.400 |
330 |
| Ca2+ |
450 |
325 |
150 |
2.400 |
547 |
2.760 |
31.000 |
4.050 |
| Sr2+ |
9 |
36 |
44 |
920 |
- |
415 |
850 |
90 |
| Ba2+ |
nd |
59 |
20 |
1.420 |
6.3 |
229 |
900 |
- |
| Anions: |
||||||||
| Cl- |
20.950 |
14.409 |
12.555 |
36.800 |
28.000 |
59.100 |
123.895 |
14.980 |
| SO42- |
2.300 |
nd |
14 |
nd |
13 |
14 |
- |
823 |
| HCO3- |
170 |
1.310 |
1.418 |
600 |
552 |
968 |
110 |
230 |
| Table 2: Solubility products Ksp of SCALE salts |
||
|---|---|---|
| Compound |
Ksp |
|
| CaSO4 |
3.73 •10-5 |
|
| SrSO4 |
3.42•0-7 |
|
| BaSO4 |
1.05•10-10 |
|
| RaSO4 |
4.30•10-11 |
|
| CaCO3 |
4.95•10-9 |
|
| FeS |
1.57•10-19 |
|
Ca2+ + SO42-→CaSO4
Ba2+ + SO42-→BaSO4
Sr2+ + SO42-→SrSO4
(Ra2+)x + (Ba2+)y + (SO42-)z→[Ba(Ra)SO4]z
where x + y = z and x ≪ y. Radium is co-precipitated with barium sulphate and the other sulphates and carbonates.
What is radioactive scale, LRA?
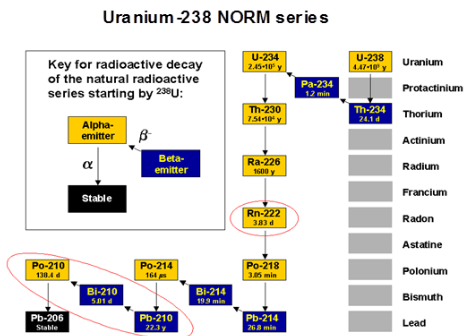
Uranium and Thorium in rocks are not easily dissolved and transported by the moving waters. Their decay products are solvable. These are Radium (226Ra from the 238U-series and 228Ra and 224Ra from the 232Th-series) and Radon ( 222Rn from the 238U-series and 220Rn from the 232Th-series). In brines with high chloride concentration also Lead isotopes (214Pb and 210Pb from the 238U-series and 212Pb from the 232Th-series) dissolve as chloride complexes and are easily transported in the water systems.These radionuclides are the main sources for radioactivity in scales, in waters, oils and gases and in internal surface coating of production equipment.
LRA may be roughly classified into two basically different types:
 |
|---|
| Fig 1: The decay of 238U |
The Radium-based LRA that is found in fields with water production. This scale is predominantly containing 226Ra and 228Ra and their progeny (may also contain some primary transported 210Pb). Since the concentration of Radium in formation waters is much too low for precipitation with available anions on its own (i.e. the solubility product is not exceeded), the radioactivity concentration in scale is due to co-precipitation with analogues alkaline earth elements present in macro-amounts. These are mainly Ca, Sr and Ba, and the main precipitates are BaSO4, SrSO4 and CaCO3 according to the general equation
xBa2+ + yRa2+ (x+y)SO42-⇄ Bax(Ra)y[SO4](x+y)
Contaminated equipment may be production tubing, valves, pumps, separators, storage tanks etc.
2. Radon-based deposit that is also found in oil-handling facilities and most importantly in gas-producing facilities. This latter deposit is composed of thin (invisible) layers of 210Pb and its decay products 210Bi and 210Po. Contaminated equipment may be those mentioned above and in addition gas transportation tubing, gas handling equipment (refineries), gas storage tanks etc.
 |
|---|
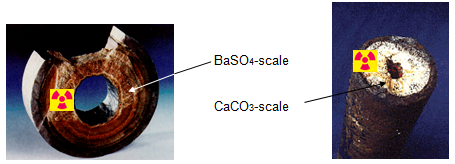
| Fig 2: Picture of scale in pipes |
Analysis of radioactive scale
Analysis of radioactivity in scale is best carried out with gamma spectroscopy. For this purpose one may for instance use NaI(Tl)-scintillation detectors. An example of the lower portion of a gamma spectrum (< 550 keV) accumulated with a NaI(Tl)-detector is given in the fig. 3.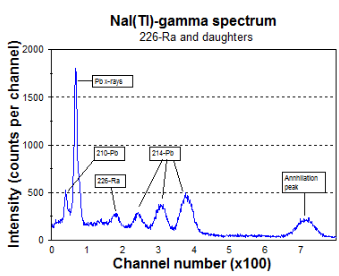 |
|---|
| Fig 3: The spectrum of 226Ra from a NaI detector |
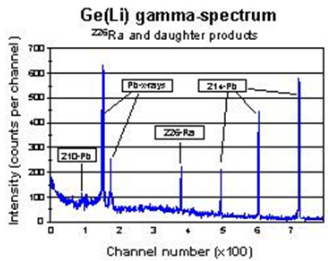
Since the gamma spectrum is relatively complex, it is recommended to use a high-resolution HpGe-detector in order to resolve the photopeaks and obtain better accuracy in the analysis. An example of a gamma spectrum accumulated with a HpGe-detector in the same energy range is given in the fig. 4.
 |
|---|
| Fig 4. Spetrum in Ge detector |
Decay schemes, gammaspectra and relevant gamma energies for NORM nuclides in radioactive scale are given as attachment to this text.
The degree of scaling in production equipment can be so heavy that flow is seriously reduced or even hindered. In such cases equipment, like production tubing in production wells have to be replaced.
The used tubing is treated specially because of the radioactivity in the scale. The first procedure on the offshore production platforms is to separate the “radioactive” from the “non-radioactive”. For this purpose, simple detection procedures have been established that are capable of deciding whether the activity concentration is below or above the exemption level.
The practiced exemption level in Norway is
10 Bq/g for 226Ra
10 Bq/g for 228Ra
10 Bq/g for 210Pb
i.e. if the activity concentration of one of these radionuclides supersedes this limit, the material will have to be treated as low-activity waste.
The scale which shows an activity concentration above the exemption level is then subject to closer and detailed analysis by gamma spectrometry in the laboratory.
In this exercise we will determine the activity concentration in examples of radioactive scale originating from the Norwegian petroleum production.